Magnesium Sulfate for Women with Preeclampsia

Benefits in NNT
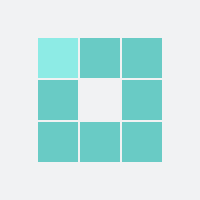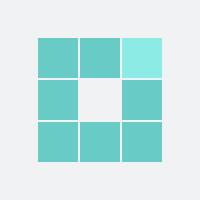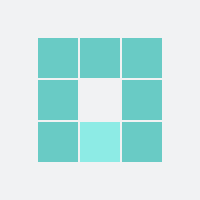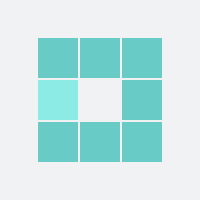
90
For the mother: 1 in 90 were helped (seizure prevented)
For the child: None were helped (death, NICU stay, avoiding preterm delivery)

Harms in NNT
200
For the mother: 1 in 200 were harmed (respiratory depression)
37
For the mother: 1 in 37 were harmed (caesarian section)
For the child: None were harmed (death, neurologic disability)
View As:
Source
Duley L, Gülmezoglu AM, Henderson-Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010 Nov 10;(11):CD000025. Review. PubMed PMID: 21069663.Weeks AD, Ononge S. The magpie trial. Lancet. 2002 Oct 26;360(9342):1331; author reply 1331-2. PubMed PMID: 12414232.
Efficacy Endpoints
Mother: Mortality, eclampsia, serious maternal morbidity related to preeclampsia (renal failure, liver failure, stroke, coagulopathy) Child: Mortality, preterm birth, NICU stay> 7 dayHarm Endpoints
Mother: Respiratory Depression, risk of cesarian section, postpartum hemorrhage (up to 2 years) Child: Mortality, neurosensory disability/ cerebral palsy (up to 18 months)Narrative
Preeclampsia is a multisystem disorder usually associated with hypertension and proteinuria. Eclampsia, or seizures in the setting of pre-eclampsia, is a rare but known neurologic complication of preeclampsia accounting for 50,000 deaths worldwide (10% direct maternal death). Although the mechanism is unclear magneisum sulfate has been used to prevent eclampsia since the 1950s.In this cochrane review, 11,444 women in 15 randomized trials were recruited from a mix of high, middle, and low income countries. The largest is the 33-country MagPie Trial comparing intravenous magnesium to placebo. The study was published in 2002 and at >10,000 subjects accounts for more than 87% of those in the review.
The use of magnesium sulfate was associated with a greater than 50% relative reduction in the risk of eclampsia (RR 0.41). As noted by the reviewers, a similar reduction in maternal mortality (RR 0.54) was also found, though the absolute number of deaths was small which may have kept this reduction from reaching statistical significance. A reduction in placental abruption was also noted (RR 0.64) as well as a small increase in the rate of cesarian section. No differences were seen in child outcomes.
As a secondary outcome, magnesium sulfate was more effective than phenytoin for reducing risk of eclampsia in 3 trials (RR 0.08) but also increased caesarian section in 2 trials (RR 1.21).
Toxicity with magnesium sulfate is rare with 0.5% of women having respiratory depression (RR 1.98). More minor side effects such as flushing (20%) were also noted.
Caveats
This review strongly supports the current use of magnesium sulfate as the drug of choice in the prevention of eclampsia, particularly in those with severe preeclampsia, and provides reasonable reassurance that this benefit in the short term is not associated with long-term negative sequelae for either women (up to 2 years) or children (up to 18 months). Although few women in these studies had mild preeclampsia, it would seem plausible that magnesium sulfate would also reduce the risk of eclampsia in these women. In addition, given the safety profile of magnesium sulfate and the ability to monitor toxicity clinically with limited training, serum monitoring is not required, allowing for wide applicability in hospital settings.Author
Nadia Shaukat, MDPublished/Updated
March 13, 2012